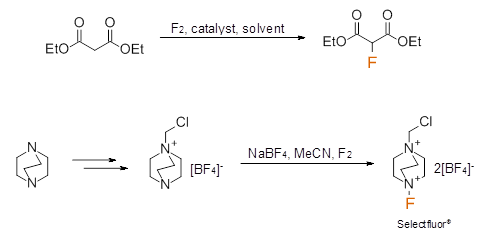
Elemental Fluorine and 'F+' sources
Elemental fluorine is an extremely reactive, oxidizing gas; it should only be used by qualified individuals with specialist equipment and appropriate failsafe measures in place. When subjecting organic molecules to fluorine gas, selectivity can be difficult to predict and control. We at Cheshire Organics, however, have considerable experience with this type of chemistry.
A direct F2 gas mediated fluorination and fluorination to furnish Selectfluor® are shown below.
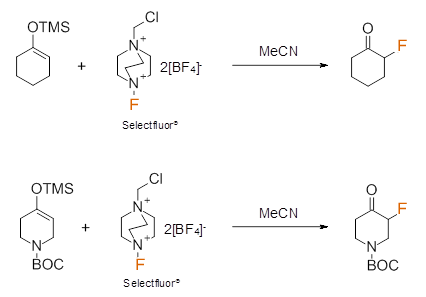
Electrophilic fluorine sources such as NFSI and Selectfluor® are also used to perform fluorinations at Cheshire Organics. These sources of ‘F+’ are much less hazardous and easier to handle than deoxofluorination reagents like DAST and SF4. They are extremely useful reagents and have been reported to effect enantioselective fluorination alpha to carbonyl groups as just one example of a wide range of reactions with nucleophiles. Whilst Selectfluor® usually requires polar solvents such as MeCN for its reactions; NFSI can be employed in more general solvent media such as THF, broadening the scope of molecules upon which this mode of fluorination can be done.
Examples of electrophilic fluorination are depicted below.